Hello friends welcome to this series of blog which explains the subject of Pharmacovigilance in a simplified manner. Let us start with the question what Pharmacovigilance all about? Before we get into the detailed discussion about Pharmacovigilance, let me put some simple questions across. We all used medicines, so what is the general thought which comes to our mind when we consume medicines thought is it will cure the disease. It will bring relief and it does happen so actually that's the objective of any medicine.
What about the side-effects of medicines?
Suppose we took the medicine for a simple headache and we start feeling nausea or some other problem. It is because medicines have side effects, but these side effects can be so serious sometimes that it can cause permanent disability or some serious health conditions or even fatality well. So now the million-dollar question is how to minimize the side effects or adverse effects related to a medicinal product, that's where Pharmacovigilance or drug safety comes in. Pharmacovigilance is something which refers to monitoring of effects of pharmaceutical products.
How can monitoring be helpful in reducing is the side effects or the adverse effects of medicines ?
When monitor we basically track the impact of medicines on the patients and it helps in highlighting if there is any alarming effect of medicine.
What is the prime objective of Pharmacovigilance?
The main objective of Pharmacovigilance is to ensure ethical, rational and safe use of my to proper monitoring of effects of medicinal products. One more thing we should mention that medicines can produce desired as well as undesired effects which we call the side effects.
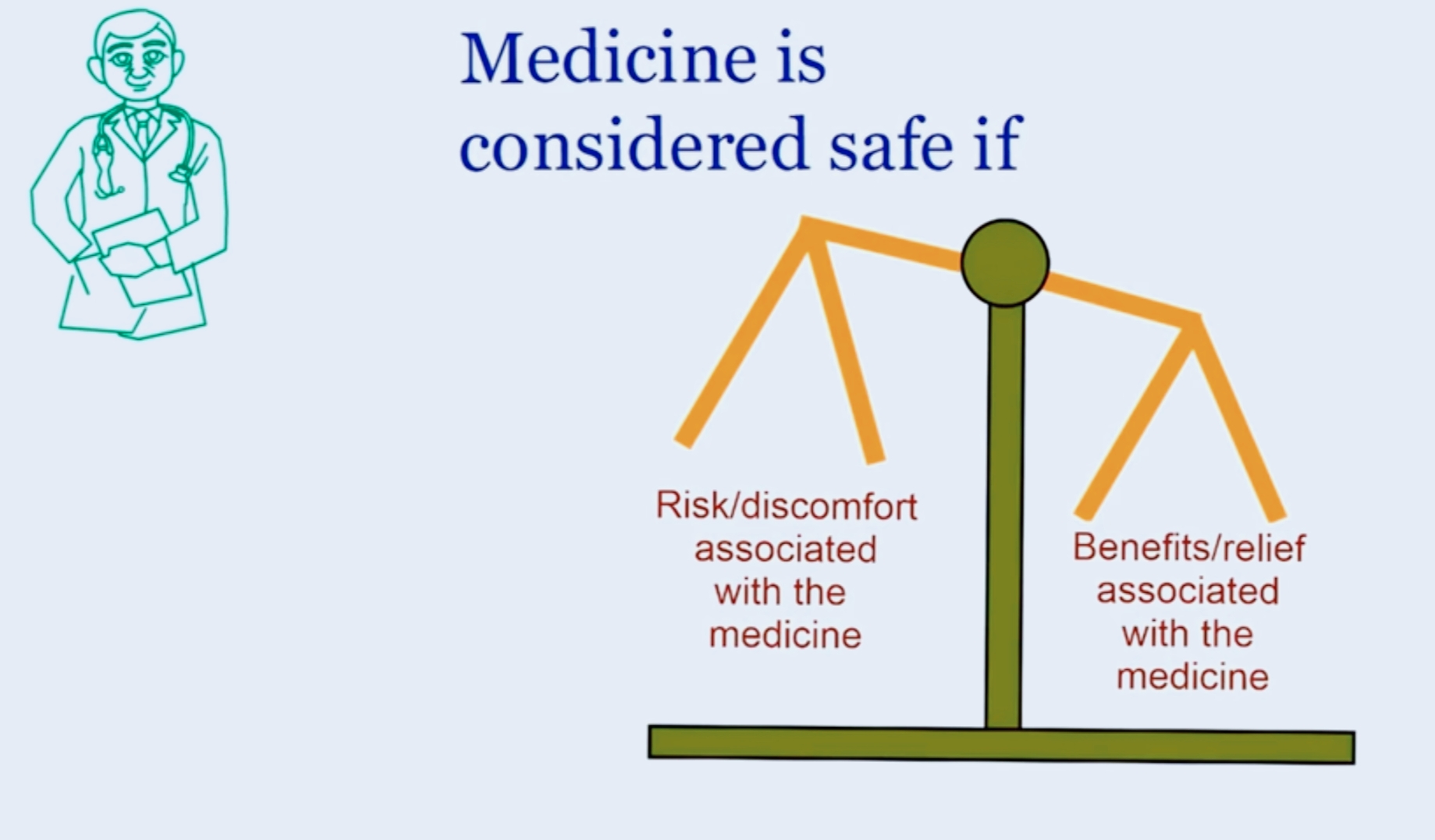
What basis does a doctor prescribe medicines to a patient?
The figure below explains that a drug is considered safe when the potential benefits of the medicine is out weighing the possible harm which may happen due to a medicine in question.
When does the drug safety monitoring actually start?
Drug monitoring is a continuous process and it covers the complete lifecycle of a drug starting from the test face to face in which the drug is available in the market for use in general problem.
Why should it be monitored post it is available for use in general public?
In terms of pre mark the safety and efficacy of the drug would have already got tested in the pre-marketing phase and only then the drug is made available for public. However, pre market study is done in a controlled environment in every aspect such as number of patients duration of experiment procedure a predetermined token and hence the information gathered related to the medicine will be limited to that extent. Suppose drug X has been studied in 3,000 patients before getting approval for marketing, but what about the rare and serious adverse effects that can be seen only in one out of six thousand patients. We can't study that adverse effect in the trial of three thousand patients. Let me further simplify every patient using a medicine has a unique environment in which the medicine can operate. Hence the medically tested in limited conditions say with just three thousand patients can provide the information of medicinal efficacy only in three thousand conditions. However, when it is made available for the whole population the scope of studying the effect of the medicine increases, many forms and a lot more information can be gathered which helps in the proper evaluation of effectiveness and safety of the drug in the larger population. Due to the same reason the health authorities demand from the manufacturers to continue this vigilance even after launching the therapeutic product for general use.
Here we can see in this figure the dots representing the scope of information collected in a controlled environment and in the general public.
I have listed a few where the post-marketing monitoring has helped in studying the medicinal effect. Elaborate the first example for more clarity Nimesulide was one of the preferred prescribe painkiller as it was having lesser events of gastritis but when it was found in post marketing studies that Nimesulide can cause liver damage it started getting banned in various countries. According to US FDA a drug is taken off from the market when the harm caused by the medicine is greater than the benefits provided with that medicine and medicine is normally removed from the market went safety issues cannot be corrected and it is a serious threat to the public health.








0 Comments
Please do not enter any spam link in the comment box.