Hello friends welcome to this series of blog which
explains the subject of Pharmacovigilance in a simplified manner. Let us start
with the question what Pharmacovigilance all about? Before we get into the
detailed discussion about Pharmacovigilance, let me put some simple questions
across. We all used medicines, so what is the general thought which comes to
our mind when we consume medicines thought is it will cure the disease. It will
bring relief and it does happen so actually that's the objective of any medicine.
What about the side-effects of medicines?
Suppose we took the medicine for a simple
headache and we start feeling nausea or some other problem. It is because
medicines have side effects, but these side effects can be so serious sometimes that it can
cause permanent disability or some serious health conditions or even fatality
well.
So now the million-dollar question is how to minimize the side effects or
adverse effects related to a medicinal product, that's where Pharmacovigilance
or drug safety comes in. Pharmacovigilance is something which refers to
monitoring of effects of pharmaceutical products.
How can monitoring be helpful in reducing is the
side effects or the adverse effects of medicines ?
When monitor we basically track the impact of
medicines on the patients and it helps
in highlighting if there is any alarming effect of medicine.
What is the prime objective of Pharmacovigilance?
The main objective
of Pharmacovigilance is to
ensure ethical,
rational and safe use of
my to proper monitoring of effects of medicinal products. One more thing
we should mention that medicines
can produce desired as well as undesired effects which we call the side
effects.
What basis does a doctor prescribe medicines to a
patient?
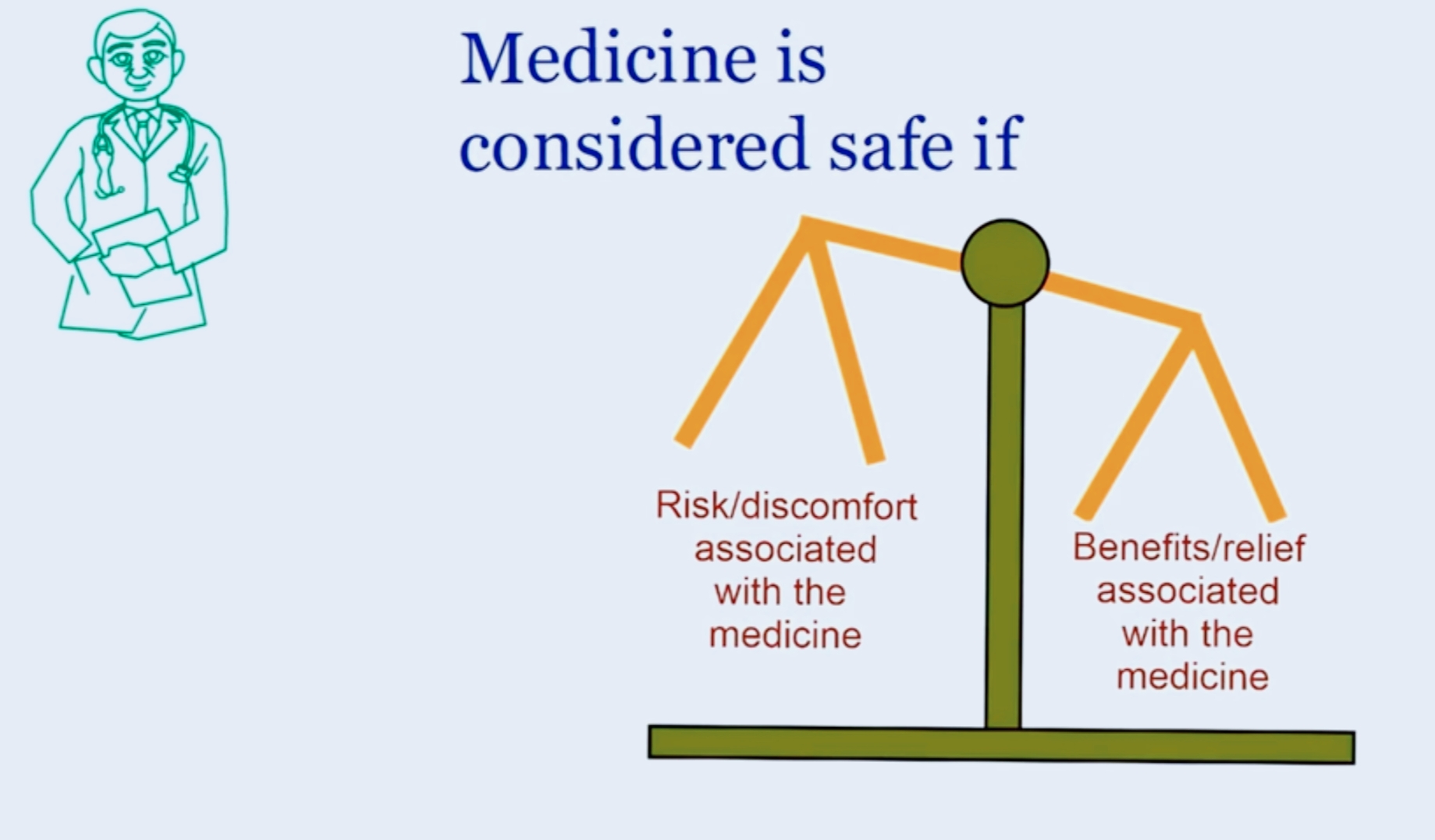
The figure below explains that a drug is considered
safe when the potential benefits of the medicine is out weighing the possible harm which may happen
due to a medicine in question.
When does the drug safety monitoring actually
start?
Drug monitoring is a continuous
process and it covers the complete lifecycle of a drug starting
from the test face to
face in which the drug is available in the market for use
in general problem.
Why should it be monitored post it is available for use in
general public?
In terms of pre mark the safety and efficacy of the drug would
have already got tested in the pre-marketing phase and only then the drug is made available for
public. However, pre market study is done in a controlled environment in every
aspect such as number of patients duration of experiment procedure a
predetermined token and hence the information gathered related to the medicine
will be limited to that extent. Suppose drug X has been studied in 3,000 patients before getting
approval for marketing, but what about the rare and serious adverse effects
that can be seen only in one out of six
thousand patients. We can't study that adverse effect in the trial of three
thousand patients. Let me further simplify every patient using a medicine has a
unique environment in which the medicine can operate. Hence the medically tested in limited conditions
say with just three thousand patients can provide the information of medicinal
efficacy only in three thousand conditions. However, when it is made available
for the whole population the scope of studying the effect of the medicine
increases, many forms and a lot more information can be gathered which helps in
the proper evaluation of effectiveness and safety of the drug in the larger
population. Due to the same reason the health authorities demand from the
manufacturers to continue this vigilance even after launching the therapeutic
product for general use.
Here we can see in this figure the dots representing the scope of
information collected in a controlled environment and in the general public.
I have listed a few where the post-marketing
monitoring has helped in studying the medicinal effect. Elaborate the first
example for more clarity Nimesulide was
one of the preferred prescribe painkiller as it was having lesser events of
gastritis but when it was found in post marketing studies that Nimesulide can
cause liver damage it started getting banned in various countries. According to
US FDA a drug is taken off from the market when the harm caused by the medicine
is greater than the benefits provided with that medicine and medicine is normally removed from the
market went safety issues cannot be
corrected and it is a serious threat to the public health.








0 Comments
Please do not enter any spam link in the comment box.